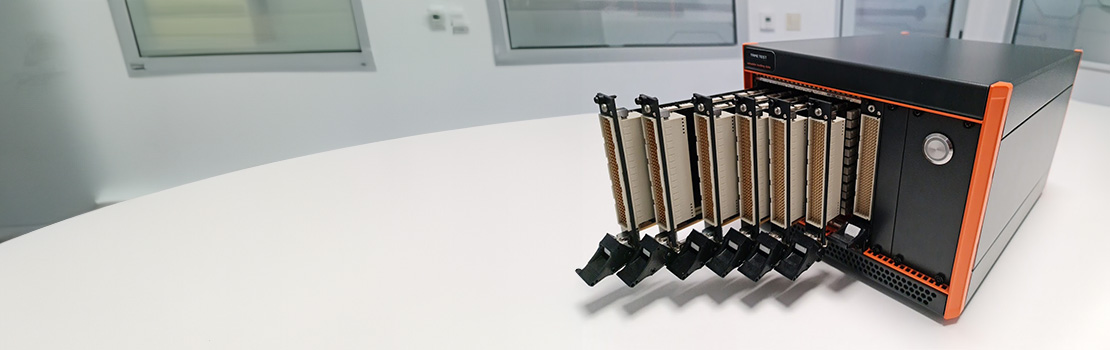
Médical
Équipements de test
Le marché du médical regroupe l’ensemble des entreprises concourant à la recherche, au développement, à la production et commercialisation dans le domaine de la santé : laboratoires pharmaceutiques, dispositifs médicaux, diagnostic in vitro et entreprises du numérique.
On retrouve sur ce marché :
- 260 laboratoires pharmaceutiques
- 1343 fabricants de dispositifs médicaux dont 92 % sont des PME.
- 100 entreprises du diagnostic in vitro dont 90 % sont des PME.
- De nombreuses start up qui innovent et porte le secteur vers le haut
- 450 biotechs et medtechs de très petite taille. Le plus souvent issues de la recherche française.
Les enjeux du marché médical
Le contexte actuel du secteur est marqué par une croissance mondiale de la demande. Les entreprises doivent continuellement innover pour rester compétitives. De plus, la concurrence étrangère est si forte qu’elle nécessite de s’adapter à une médecine toujours plus personnalisée et à des exigences réglementaires et sanitaires accrus.
Ce domaine étant extrêmement réglementé et régulé, la plupart des prix sont fixés par l’État. La position des entreprises sur le marché mondial dépend donc du maintien d’un haut niveau d’expertise technologique et du renforcement de la compétitivité des acteurs nationaux.
Les grands enjeux de demain pour ce secteur économique sont liés aux technologies médicales multiple, diagnostics et des thérapies plus innovants que des données plus faciles à extraire, notamment grâce aux objets artificiels et connectés.
Les expertises de Tame-Test
TRONICO est l’acteur de référence sur toutes les phases de vie des dispositifs médicaux hautement critiques en Europe, et Tame-Test est un pièce maitresse de cette stratégie.
Tame-Test vous assiste dans le process de fabrication de vos dispositif médicaux de classe IIa, IIb, III et DMIA.
Certifications et accréditations de Tame-Test sur le marché médical
Tame-Test conçoit et industrialise des bancs de test pour le secteur du médical qui impose, en plus du respect des normes ISO, des garanties liées à la confidentialité.
Tame-Test assure pour tout ses équipements :
- La conformité aux directives basses tensions
- Le marquage CE
- Le marquage UL
- La directive machine
De plus, si l’équipement est un accessoire de dispositif médical, Tame-Test assure la conformité aux exigences réglementaires (classe A,B ou C)
ISO 13485
L’ISO 13485 est une norme internationale de management de la qualité qui permet la mise en oeuvre des exigences réglementaires applicables aux dispositifs médicaux et services associés (DIR 93/42/CEE, DIR 98/79/CE, DIR 2007/47/CEE et DIR 90/385/CEE).
Cas d'usage
Cas d'usage n°1 : test de dispositif médical
Stéphane, fondateur d’une startup médicale, est l’inventeur d’un dispositif médical répondant à l’insuffisance cardiaque. Il aborde la phase de mise en production de son Dispositif Médical et désire que son produit soit irréprochable. Son produit intègre 3 types de cartes différentes.
Après avoir convergé avec Stéphane sur la stratégie de test, Tame-Test lui propose un testeur Check Versatile capable d’accueillir 4 interfaces, une par référence de carte et une pour le produit assemblé. Stéphane demande, après quelques semaines et suite à son retour d’expérience des premiers essais cliniques, d’implémenter un essai en inclinaison afin de calibrer des accéléromètres. Tame-Test, en ne changeant rien au testeur Versatile de départ, développe une 5ème interface équipée de mouvements mécaniques précis afin de répondre au besoin de Stéphane. Ce test est pris en charge par le testeur existant par cette nouvelle interface.